HTA III. 2021
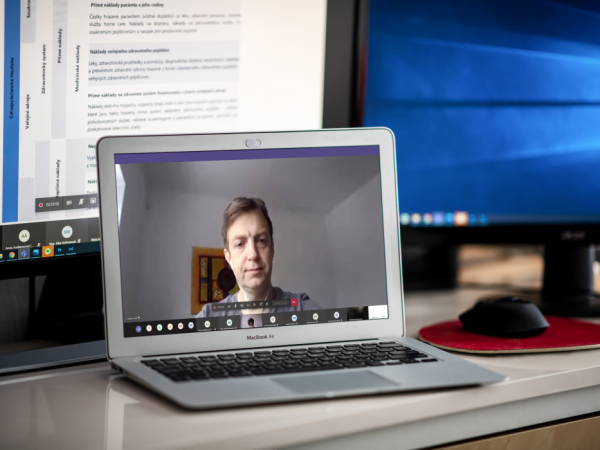
At the beginning of 2021, on Saturday 16th January, further patient organizations representatives’ education in the area of HTA took place and their involvement in health care decision-making processes. Training was online via the Teams platform.
The morning block was focused on the challenges of legislative changes for patient representatives.
The first part contained:
- What are the legislative requirements and where is the role of patients
- Measuring the quality of life and what is the significance of this parameter in HTA
- Lecturer: MUDr. Jana Skoupá, MBA (member of the committee of the Czech Pharmacoeconomic Society)
The second part contained:
- Global view on diseases (indirect and social costs, impacts on the family and carers)
- How to organize methodology of data collections correctly
- Lecturer: MUDr. Tomáš Doležal, PhD. (the Chairman of the Czech Pharmacoeconomic Society)
In the afternoon, practically focused block, the participants were divided into two groups:
- Oncology medicines with MUDr. Jana Skoupá, MBA (member of the committee of the Czech Pharmacoeconomic Society)
- Medicines for rare diseases with MUDr. Tomáš Doležal, PhD. (the Chairman of the Czech Pharmacoeconomic Society)
Photos:
No. 1 - group photo
No. 2 - introductory word by Mgr. Jakub Dvořáček, MHA, LL.M. the Executive Director of the AIFP
No. 3 - MUDr. Jana Skoupá, MBA, member of the committee of the Czech Pharmacoeconomic Society
No. 4 - MUDr. Tomáš Doležal, PhD., the Chairman of the Czech Pharmacoeconomic Society